Without certain COVID-19 cure, Philippines tries these drugs to treat patients | ABS-CBN

Welcome, Kapamilya! We use cookies to improve your browsing experience. Continuing to use this site means you agree to our use of cookies. Tell me more!
Without certain COVID-19 cure, Philippines tries these drugs to treat patients
Without certain COVID-19 cure, Philippines tries these drugs to treat patients
Kristine Sabillo,
ABS-CBN News
Published May 11, 2020 07:08 PM PHT
|
Updated May 12, 2020 06:20 AM PHT
MANILA — Almost four months since the COVID-19 virus was first identified in China, the world has yet to discover a cure or vaccine.
The World Health Organization said it’ll take at least one year to develop and distribute a vaccine.
MANILA — Almost four months since the COVID-19 virus was first identified in China, the world has yet to discover a cure or vaccine.
The World Health Organization said it’ll take at least one year to develop and distribute a vaccine.
In the meantime, medical experts have been using off-label drugs to treat severe and critical cases of COVID-19. Off-label drugs are medicine developed to treat other illnesses but are believed or have shown some evidence of effectivity against the novel coronavirus.
In the meantime, medical experts have been using off-label drugs to treat severe and critical cases of COVID-19. Off-label drugs are medicine developed to treat other illnesses but are believed or have shown some evidence of effectivity against the novel coronavirus.
Some of these drugs have been included in the WHO’s Solidarity Trial, a multi-country clinical trial in search for a COVID-19 cure. The Philippines is among the more than 200 countries participating.
Some of these drugs have been included in the WHO’s Solidarity Trial, a multi-country clinical trial in search for a COVID-19 cure. The Philippines is among the more than 200 countries participating.
Below are the list of experimental drugs being used to doctors in the Philippines and in other countries:
Below are the list of experimental drugs being used to doctors in the Philippines and in other countries:
ADVERTISEMENT
1. CHLOROQUINE AND HYDROXYCHLOROQUINE
Synthesized in 1934, Chloroquine became a major anti-malaria drug after World War II.
Synthesized in 1934, Chloroquine became a major anti-malaria drug after World War II.
The WHO included the two closely-related drugs in its Solidarity Trial after small studies in China and France showed the “possible benefit of chloroquine phosphate against pneumonia caused by COVID-19.”
The WHO included the two closely-related drugs in its Solidarity Trial after small studies in China and France showed the “possible benefit of chloroquine phosphate against pneumonia caused by COVID-19.”
However, there have been a lot of negative news about the drug as they have been tied to several side effects, including heart rhythm issues.
However, there have been a lot of negative news about the drug as they have been tied to several side effects, including heart rhythm issues.
Countries like the United States and Canada have also issued warnings against its use without supervision of a physician.
Countries like the United States and Canada have also issued warnings against its use without supervision of a physician.
Both are reportedly being used in Philippine hospitals but under close monitoring by doctors.
Both are reportedly being used in Philippine hospitals but under close monitoring by doctors.
2. REMDESIVIR
Remdesivir is an antiviral drug administered intravenously. It was originally made to treat the deadly Ebola virus. In including it in the Solidarity Trial, the WHO cited promising animal studies using remdesivir for the Middle East Respiratory Syndrome (MERS-CoV) and the severe acute respiratory syndrome (SARS), two other strains of coronavirus.
Remdesivir is an antiviral drug administered intravenously. It was originally made to treat the deadly Ebola virus. In including it in the Solidarity Trial, the WHO cited promising animal studies using remdesivir for the Middle East Respiratory Syndrome (MERS-CoV) and the severe acute respiratory syndrome (SARS), two other strains of coronavirus.
The US National Institute of Allergy and Infectious Diseases said its trial involving 1,000 people showed that patients recovered faster using remdesivir.
The US National Institute of Allergy and Infectious Diseases said its trial involving 1,000 people showed that patients recovered faster using remdesivir.
The drug has had mixed results though as a smaller study showed that it failed in curing COVID-19 patients.
The drug has had mixed results though as a smaller study showed that it failed in curing COVID-19 patients.
3. LOPINAVIR/RITONAVIR
The drug combination of lopinavir and ritonavir is a licensed treatment for HIV. Like remdesivir, they have shown positive effects on MERS and SARS patients.
The drug combination of lopinavir and ritonavir is a licensed treatment for HIV. Like remdesivir, they have shown positive effects on MERS and SARS patients.
Anecdotal evidence of their effectivity against COVID-19 were recorded in Thailand.
Anecdotal evidence of their effectivity against COVID-19 were recorded in Thailand.
“While there are indications from laboratory experiments that this combination may be effective against COVID-19, studies done so far in COVID-19 patients have been inconclusive,” WHO said, explaining the need to pursue further studies through the Solidarity Trial.
“While there are indications from laboratory experiments that this combination may be effective against COVID-19, studies done so far in COVID-19 patients have been inconclusive,” WHO said, explaining the need to pursue further studies through the Solidarity Trial.
Although recent studies showed no effect on seriously ill patients.
Although recent studies showed no effect on seriously ill patients.
Lopinavir and ritonavir are included in the WHO’s list of essential medicines although some side effects include diarrhea, nausea, vomiting, and liver damage.
Lopinavir and ritonavir are included in the WHO’s list of essential medicines although some side effects include diarrhea, nausea, vomiting, and liver damage.
4. INTERFERON BETA
Interferon beta-1a is an injectable drug used to treat multiple sclerosis, a disease that severely affects the brain and the spinal cord or central nervous system.
Interferon beta-1a is an injectable drug used to treat multiple sclerosis, a disease that severely affects the brain and the spinal cord or central nervous system.
Under the Solidarity Trial, interferon beta is administered with the drug combination of lopinavir and ritonavir.
Under the Solidarity Trial, interferon beta is administered with the drug combination of lopinavir and ritonavir.
A recently published study from Hong Kong showed that patients treated with the closely related interferon beta-1b, lopinavir/ritonavir plus the drug ribavirin, which is used to treat hepatitis, were able to recover faster from the COVID-19 illness.
A recently published study from Hong Kong showed that patients treated with the closely related interferon beta-1b, lopinavir/ritonavir plus the drug ribavirin, which is used to treat hepatitis, were able to recover faster from the COVID-19 illness.
The researchers also said the trio drug combination can reduce the quantity of viral shedding or when the virus is detectable and potentially transmissible.
The researchers also said the trio drug combination can reduce the quantity of viral shedding or when the virus is detectable and potentially transmissible.
Some patients were not initially given interferon beta-1b because of possible inflammation. However, the study showed that the groups that did not take interferon took longer to clear the virus, suggesting that interferon might be crucial in shortening the course of the illness.
Some patients were not initially given interferon beta-1b because of possible inflammation. However, the study showed that the groups that did not take interferon took longer to clear the virus, suggesting that interferon might be crucial in shortening the course of the illness.
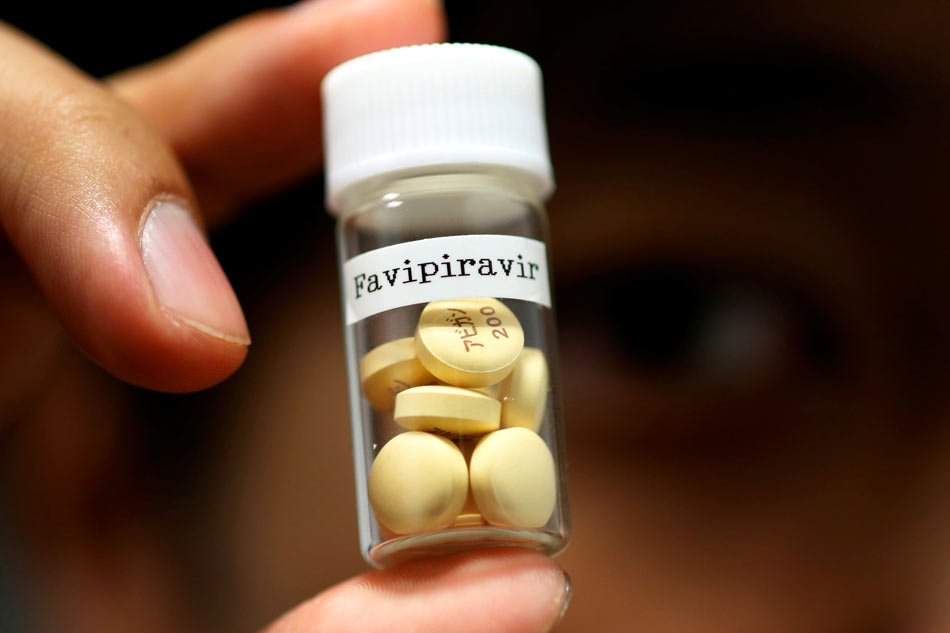
5. FAVIPIRAVIR
Just recently, the Philippines announced that it will run clinical trials on the anti-flu drug favipiravir, sold under the brand name Avigan.
Just recently, the Philippines announced that it will run clinical trials on the anti-flu drug favipiravir, sold under the brand name Avigan.
Japan has promised to provide supplies to the Philippines and 40 other countries looking for COVID-19 treatment.
Japan has promised to provide supplies to the Philippines and 40 other countries looking for COVID-19 treatment.
China has said it will include Avigan in its official list of drugs that are used to treat COVID-19 patients after clinical trials in Japan showed “encouraging results.”
China has said it will include Avigan in its official list of drugs that are used to treat COVID-19 patients after clinical trials in Japan showed “encouraging results.”
However, it is believed to be dangerous for pregnant women as it can cause birth defects.
However, it is believed to be dangerous for pregnant women as it can cause birth defects.
6. TOCILIZUMAB
The immunosuppressive drug Tocilizumab, which has been used to treat rheumatoid arthritis, is included in the Philippine Society for Microbiology and Infectious Diseases’ (PSMID) guidelines for hospital care for COVID-19 patients.
The immunosuppressive drug Tocilizumab, which has been used to treat rheumatoid arthritis, is included in the Philippine Society for Microbiology and Infectious Diseases’ (PSMID) guidelines for hospital care for COVID-19 patients.
The drug, which also goes by the brand name Actemra, is believed to help treat inflammation in patients with the coronavirus disease or those with severe immune system reactions called cytokine storms.
The drug, which also goes by the brand name Actemra, is believed to help treat inflammation in patients with the coronavirus disease or those with severe immune system reactions called cytokine storms.
Swiss drugmaker Roche hopes to finish its clinical trials on Actemra by May or June.
Swiss drugmaker Roche hopes to finish its clinical trials on Actemra by May or June.
7. FAMOTIDINE
One study that has not yet been peer-reviewed reportedly shows the effectivity of heartburn medicine famotidine against COVID-19.
One study that has not yet been peer-reviewed reportedly shows the effectivity of heartburn medicine famotidine against COVID-19.
Famotidine is an active ingredient in the hearburn treatment Pepcid. The study found that those who took the drug had decreased risk of dying or being intubated. However, the researchers said it is still not clear why and that the findings “should not be interpreted to mean that famotidine improves outcomes in patients hospitalized with COVID-19.”
Famotidine is an active ingredient in the hearburn treatment Pepcid. The study found that those who took the drug had decreased risk of dying or being intubated. However, the researchers said it is still not clear why and that the findings “should not be interpreted to mean that famotidine improves outcomes in patients hospitalized with COVID-19.”
OTHER DRUGS
Other off-label drugs that are being studied are Sarilumab or Kevzara, which was developed for rheumatoid arthritis; Azithromycin, which has been used to treat various bacterial infections such as strep throat and pneumonia; Umifenovir or Arbidol, an antiviral treatment for influenza; Ciclesonide, an inhaled corticosteroid for asthma; Colchicine, a drug used to treat gout and is believed to help those with mild symptoms; Dipyridamole, a drug that inhibits blood clot formation; and even Sildenafil or Viagra, which has been used to treat erectile dysfunction and pulmonary arterial hypertension.
Other off-label drugs that are being studied are Sarilumab or Kevzara, which was developed for rheumatoid arthritis; Azithromycin, which has been used to treat various bacterial infections such as strep throat and pneumonia; Umifenovir or Arbidol, an antiviral treatment for influenza; Ciclesonide, an inhaled corticosteroid for asthma; Colchicine, a drug used to treat gout and is believed to help those with mild symptoms; Dipyridamole, a drug that inhibits blood clot formation; and even Sildenafil or Viagra, which has been used to treat erectile dysfunction and pulmonary arterial hypertension.
Read More:
COVID-19
coronavirus
experimental drugs
off-label drugs
COVID-19 treatment
COVID-19 cure
remdesivir
chloroquine
hydroxychloroquine
avigan
ADVERTISEMENT
ADVERTISEMENT