FDA forms task force to hasten release of COVID-19 drugs in Philippine markets | ABS-CBN

Welcome, Kapamilya! We use cookies to improve your browsing experience. Continuing to use this site means you agree to our use of cookies. Tell me more!
FDA forms task force to hasten release of COVID-19 drugs in Philippine markets
FDA forms task force to hasten release of COVID-19 drugs in Philippine markets
ABS-CBN News
Published Feb 24, 2023 02:52 PM PHT
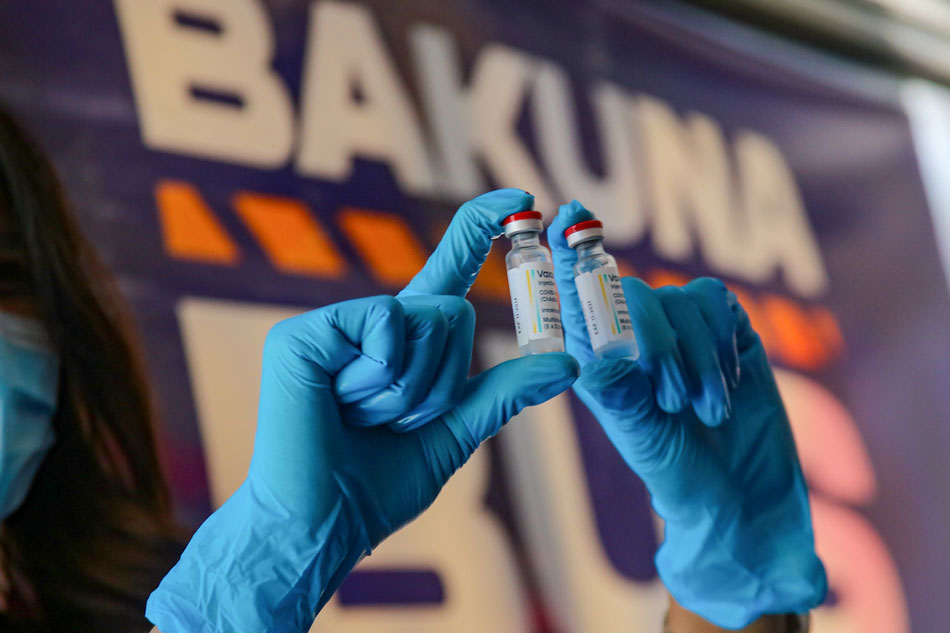
MANILA — The Food and Drug Administration has created a task force to hasten the release of COVID-19 drugs in the Philippine market.
MANILA — The Food and Drug Administration has created a task force to hasten the release of COVID-19 drugs in the Philippine market.
According to FDA Director General Samuel Zacate, Task Force Fleming will streamline the approval and evaluation of COVID-19 drugs "without compromising efficacy, quality and safety".
According to FDA Director General Samuel Zacate, Task Force Fleming will streamline the approval and evaluation of COVID-19 drugs "without compromising efficacy, quality and safety".
The state drug regulator has issued emergency use authorizations for COVID-19 drugs and vaccines with certain conditions.
The state drug regulator has issued emergency use authorizations for COVID-19 drugs and vaccines with certain conditions.
"Now, with the introduction of Task Force Fleming, COVID-19 drugs that will be approved and issued with Certificates of Product Registration will be readily accessible to the public in FDA-licensed drug establishments with the assurance that any post-market issues will be addressed through more rigorous surveillance and pharmacovigilance," Zacate said in a statement.
"Now, with the introduction of Task Force Fleming, COVID-19 drugs that will be approved and issued with Certificates of Product Registration will be readily accessible to the public in FDA-licensed drug establishments with the assurance that any post-market issues will be addressed through more rigorous surveillance and pharmacovigilance," Zacate said in a statement.
ADVERTISEMENT
To date, 4 COVID-19 drug EUA holders have submitted their applications for a CPR.
To date, 4 COVID-19 drug EUA holders have submitted their applications for a CPR.
Zacate urged pharmaceutical companies to apply for a CPR.
Zacate urged pharmaceutical companies to apply for a CPR.
Department of Health officer-in-charge Maria Rosario Vergeire also made a similar appeal.
Department of Health officer-in-charge Maria Rosario Vergeire also made a similar appeal.
"We welcome FDA’s efforts to institutionalize this initiative that will focus on processing the registration of COVID-19 drugs and to make them available commercially," she said in a statement.
"We welcome FDA’s efforts to institutionalize this initiative that will focus on processing the registration of COVID-19 drugs and to make them available commercially," she said in a statement.
"We also encourage manufacturers or suppliers to transition their COVID-19 EUAs into a product registration as this will be a good start to our economic recovery."
"We also encourage manufacturers or suppliers to transition their COVID-19 EUAs into a product registration as this will be a good start to our economic recovery."
ADVERTISEMENT
As of February 23, the Philippines has 9,269 active COVID-19 cases.
As of February 23, the Philippines has 9,269 active COVID-19 cases.
Since the pandemic started, the country has recorded over 4 million coronavirus infections, of which 66,000 resulted in deaths.
Since the pandemic started, the country has recorded over 4 million coronavirus infections, of which 66,000 resulted in deaths.
To date, more than 73.8 million Filipinos are fully vaccinated against COVID-19.
To date, more than 73.8 million Filipinos are fully vaccinated against COVID-19.
Of the figure, some 21.5 million people have received their first boosters while 3.9 million have gotten their second boosters.
Of the figure, some 21.5 million people have received their first boosters while 3.9 million have gotten their second boosters.
RELATED VIDEO
Read More:
coronavirus
health
Food and Drug Administration
FDA
Department of Health
DOH
COVID-19
COVID19
virus
disease
ADVERTISEMENT
ADVERTISEMENT