FDA: AstraZeneca has assured PH COVID-19 vaccine safe amid blood clot concerns
ADVERTISEMENT

Welcome, Kapamilya! We use cookies to improve your browsing experience. Continuing to use this site means you agree to our use of cookies. Tell me more!
FDA: AstraZeneca has assured PH COVID-19 vaccine safe amid blood clot concerns
Kristine Sabillo,
ABS-CBN News
Published Mar 18, 2021 02:45 PM PHT
MANILA — The Food and Drug Administration on Thursday said pharmaceutical firm AstraZeneca has assured the Philippine government that its COVID-19 vaccine is safe and is unrelated to blood clotting incidents reported in Europe.
MANILA — The Food and Drug Administration on Thursday said pharmaceutical firm AstraZeneca has assured the Philippine government that its COVID-19 vaccine is safe and is unrelated to blood clotting incidents reported in Europe.
“'Yung AstraZeneca keeps us updated on what is happening tulad ng nangyayari sa (like what is happening in) Europe,” FDA Director General Eric Domingo said during a virtual media briefing.
“'Yung AstraZeneca keeps us updated on what is happening tulad ng nangyayari sa (like what is happening in) Europe,” FDA Director General Eric Domingo said during a virtual media briefing.
“We had a meeting last night and findings of the blood clotting events are similar to what would happen in a normal population without the vaccine or in populations vaccinated even with other brands,” he said.
“We had a meeting last night and findings of the blood clotting events are similar to what would happen in a normal population without the vaccine or in populations vaccinated even with other brands,” he said.
The Philippine government had already announced that it would continue using the AstraZeneca coronavirus vaccine, one of the only two vaccines currently available in the country. The country has so far received 525,600 doses from AstraZeneca and 600,000 doses from Sinovac.
The Philippine government had already announced that it would continue using the AstraZeneca coronavirus vaccine, one of the only two vaccines currently available in the country. The country has so far received 525,600 doses from AstraZeneca and 600,000 doses from Sinovac.
ADVERTISEMENT
Duque previously said that the company has not seen any evidence linking the vaccine to reports of blood clotting.
Duque previously said that the company has not seen any evidence linking the vaccine to reports of blood clotting.
"A careful review of all available safety data of more than 17 million people vaccinated in the European Union and UK with COVID-19 Vaccine AstraZeneca has shown no evidence of an increased risk of pulmonary embolism, deep vein thrombosis or thrombocytopenia, in any defined age group, gender, batch or in any particular country," the company said in a statement issued over the weekend.
"A careful review of all available safety data of more than 17 million people vaccinated in the European Union and UK with COVID-19 Vaccine AstraZeneca has shown no evidence of an increased risk of pulmonary embolism, deep vein thrombosis or thrombocytopenia, in any defined age group, gender, batch or in any particular country," the company said in a statement issued over the weekend.
Countries like Ireland, Denmark, Norway and Iceland have suspended the use of the vaccine as they probe deaths reportedly due to coagulation disorders.
Countries like Ireland, Denmark, Norway and Iceland have suspended the use of the vaccine as they probe deaths reportedly due to coagulation disorders.
Besides the doses from the COVAX Facility that arrived in the country, the private sector and local government units also ordered 17 million doses from AstraZeneca. An initial batch of 2.6 millions is expected to arrive in May, according to presidential adviser for entrepreneurship Joey Concepcion.
Besides the doses from the COVAX Facility that arrived in the country, the private sector and local government units also ordered 17 million doses from AstraZeneca. An initial batch of 2.6 millions is expected to arrive in May, according to presidential adviser for entrepreneurship Joey Concepcion.
AstraZeneca is one of the three companies with emergency use authorization for COVID-19 vaccines in the Philippines. The other two are Sinovac and Pfizer.
AstraZeneca is one of the three companies with emergency use authorization for COVID-19 vaccines in the Philippines. The other two are Sinovac and Pfizer.
ADVERTISEMENT
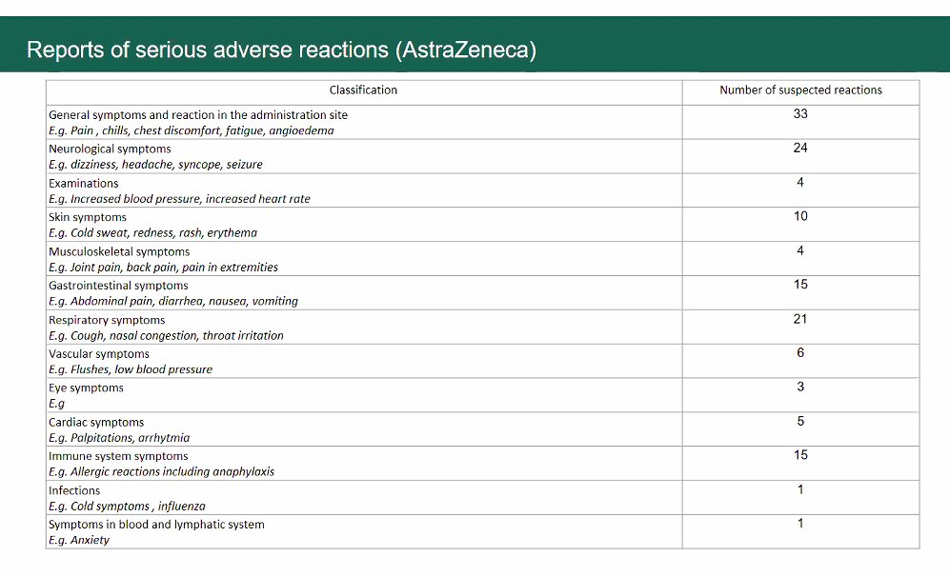
As of March 17, more than 70,000 health workers have been inoculated with AstraZeneca’s vaccine. The FDA said there were 3,769 suspected adverse events reported by those who received the jabs, representing 5.19% of those vaccinated.
As of March 17, more than 70,000 health workers have been inoculated with AstraZeneca’s vaccine. The FDA said there were 3,769 suspected adverse events reported by those who received the jabs, representing 5.19% of those vaccinated.
FDA Director General Eric Domingo said this is within the expected range since clinical trials often monitor up to 30% reports of adverse events.
FDA Director General Eric Domingo said this is within the expected range since clinical trials often monitor up to 30% reports of adverse events.
Most of the reported reactions are body pain, fever, headache, nasal congestion and other common vaccine side effects.
Most of the reported reactions are body pain, fever, headache, nasal congestion and other common vaccine side effects.
RELATED VIDEO
Read More:
COVID-19 vaccine
COVID-19 vaccine in the Philippines
AstraZeneca vaccine
blood clotting
Department of Health
Food and Drug Administration
ADVERTISEMENT
ADVERTISEMENT