PH signs deal to secure 30 million doses of COVID-19 vaccine Covovax | ABS-CBN
ADVERTISEMENT

Welcome, Kapamilya! We use cookies to improve your browsing experience. Continuing to use this site means you agree to our use of cookies. Tell me more!
PH signs deal to secure 30 million doses of COVID-19 vaccine Covovax
PH signs deal to secure 30 million doses of COVID-19 vaccine Covovax
Reuters
Published Jan 11, 2021 06:49 AM PHT
|
Updated Jan 11, 2021 09:50 AM PHT
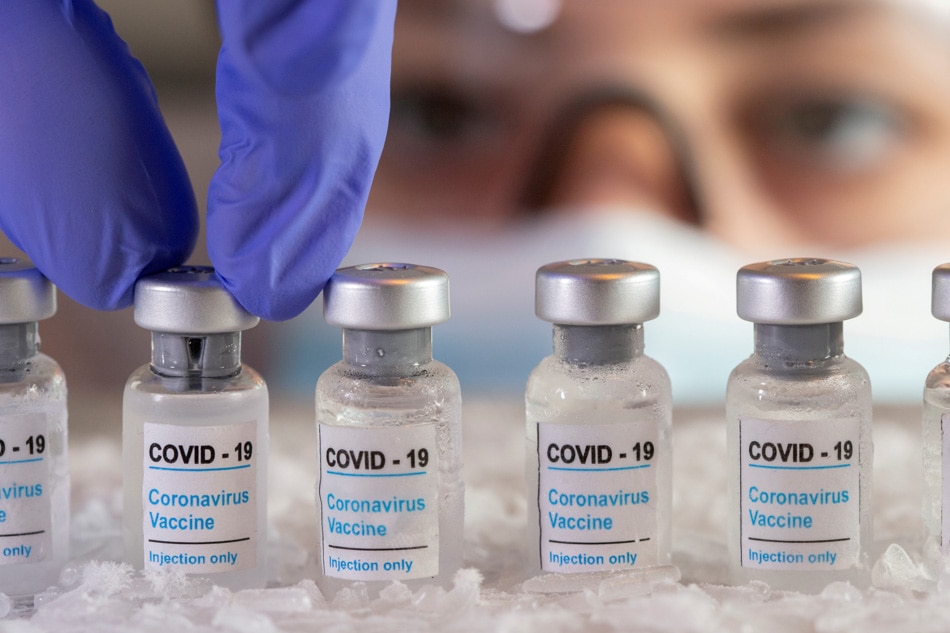
MANILA (UPDATE) - The Philippine government has signed a deal to secure the supply of 30 million doses of the COVID-19 vaccine Covovax from Serum Institute of India (SII), the latter's local partner said on Sunday.
MANILA (UPDATE) - The Philippine government has signed a deal to secure the supply of 30 million doses of the COVID-19 vaccine Covovax from Serum Institute of India (SII), the latter's local partner said on Sunday.
The agreement was signed on Saturday by Carlito Galvez, a former military general in charge of the Philippines' strategy to fight the coronavirus, according to a statement issued by SII's local partner, Faberco Life Sciences Inc.
The agreement was signed on Saturday by Carlito Galvez, a former military general in charge of the Philippines' strategy to fight the coronavirus, according to a statement issued by SII's local partner, Faberco Life Sciences Inc.
The vaccine is kept at a temperature of "2 to 8 degrees centigrade," according to Faberco medical director Dr. Luningning Villa.
The vaccine is kept at a temperature of "2 to 8 degrees centigrade," according to Faberco medical director Dr. Luningning Villa.
"Di po tayo nangangailangan ng ultra-low temperature, pwede po siya kahit sa mga malalayong lugar," she told ABS-CBN's Teleradyo.
"Di po tayo nangangailangan ng ultra-low temperature, pwede po siya kahit sa mga malalayong lugar," she told ABS-CBN's Teleradyo.
ADVERTISEMENT
(We don't need to store it in ultra-low temperature, it can be distributed to even the farthest areas.)
(We don't need to store it in ultra-low temperature, it can be distributed to even the farthest areas.)
The Department of Health, which helped distribute the Faberco statement to local media, has yet to issue its own statement. Galvez could not be immediately reached for comment.
The Department of Health, which helped distribute the Faberco statement to local media, has yet to issue its own statement. Galvez could not be immediately reached for comment.
Health Secretary Francisco Duque in a tweet said: "We're in the final stages of closing agreements with various manufacturers to vaccinate at least 60-70% of the (population)". He gave no further details.
Health Secretary Francisco Duque in a tweet said: "We're in the final stages of closing agreements with various manufacturers to vaccinate at least 60-70% of the (population)". He gave no further details.
SII partnered with US-based Novavax Inc for the development and commercialization of Covovax, which is in third-stage trials and expected to be approved for use by international regulators, Faberco said.
SII partnered with US-based Novavax Inc for the development and commercialization of Covovax, which is in third-stage trials and expected to be approved for use by international regulators, Faberco said.
The vaccine will be available locally by the third quarter of 2021 and will be used to inoculate 15 million vulnerable and poor Filipinos, it said.
The vaccine will be available locally by the third quarter of 2021 and will be used to inoculate 15 million vulnerable and poor Filipinos, it said.
ADVERTISEMENT
Galvez has said the Philippines was negotiating with seven vaccine manufacturers to procure at least 148 million COVID-19 shots as it seeks to inoculate close to two-thirds of its population this year.
Galvez has said the Philippines was negotiating with seven vaccine manufacturers to procure at least 148 million COVID-19 shots as it seeks to inoculate close to two-thirds of its population this year.
Manila is hoping to close similar deals with Moderna , AstraZeneca, Pfizer, Johnson & Johnson , Sinovac Biotech and the Gamaleya Institute this month.
Manila is hoping to close similar deals with Moderna , AstraZeneca, Pfizer, Johnson & Johnson , Sinovac Biotech and the Gamaleya Institute this month.
There are now three applicants for emergency use authorization of their vaccines in the Philippines - Pfizer, AstraZeneca, and Russia's Gamaleya.
There are now three applicants for emergency use authorization of their vaccines in the Philippines - Pfizer, AstraZeneca, and Russia's Gamaleya.
With total confirmed COVID-19 infections of 487,690 and deaths reaching 9,405, the Philippines has the second-highest number of cases and fatalities in Southeast Asia, after Indonesia.
With total confirmed COVID-19 infections of 487,690 and deaths reaching 9,405, the Philippines has the second-highest number of cases and fatalities in Southeast Asia, after Indonesia.
--with reports from ABS-CBN News
Read More:
ANC
COVID-19 vaccine
Covovax
Novovax
Philippines COVID-19 vaccination
Philippines COVID-19
COVID-19
coronavirus
Teleradyo
Luningning Villa
ADVERTISEMENT
ADVERTISEMENT